Deci liter or gram sugar The SI derived unit for volume is the cubic meter. Shows how to use stoichiometry to convert from grams of a substance to liters of a substanceA chemical reaction is a process that leads to the chemical chan.

Chemical Reactions 11 Of 11 Stoichiometry Grams To Liters Of A Gas Youtube
1 cubic meter is equal to 10000 deci liter or 85211336848478 gram sugar.
Convert liters to grams chemistry. Because the molar volume is the same. There is no conversion formula between the two units. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
Its chemistry and its giving me differents substances like CO2 and oxygen and hydrogen. Quick conversion chart of liters to grams 1 liters to grams 1000 grams. Converting from mass.
L to g conversion table. 1 gram g 0001 liter l. The answer is 001173552765377.
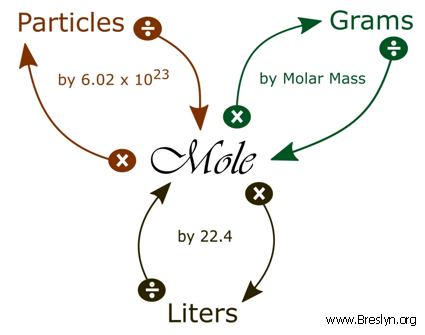
Please note this is weight to volume conversion this conversion is valid only for pure water at temperature 4 C. Hence 1 liter is equal to 1 kilogram or 1000 grams. Going from liters to grams you divide by the 224 Lmol and then multiply by the molar mass.
Please note this is volume to weight conversion this conversion is valid only for pure water at temperature 4 C. This online calculator converts grams to liters and liters to grams given a gas formula. Gram g is a unit of Weight used in the Metric system.
Convert grams to liters and liters to grams. Gram g is a unit of Weight used in Metric system. To convert to gL you have to calculate mass of solute using density Consider 15vv ethanol Density of ethanol 0879gmL Mass of 15 mL ethanol 15mL 0879gmL 132g You then have a 132mv solution of ethanol - which as before is 132gL.
You can view more details on each measurement unit. Instead you must use atomic mass values and the chemical formula to do the conversion. It uses molar volume of a gas at STP standard temperature and pressure person_outlineTimurschedule 2018-09-17 104101.
To convert 12 liters to g we use the formula L 12 x 1000 D. You can find several conversion calculators on the Internet. As an example converting 10 liters into grams is done as follows.
Please note this is volume to weight conversion this conversion is Read more. 10 liters 10 x 1000 milliliters Specific unit weight of concrete - amount properties converter for conversion factor exchange from 1 liter L equals 240653 grams g exactly for the masonry material type. Use this page to learn how to convert between grams and liters.
Under these circumstances 12 liters of water equal 1200 grams. More information from the unit converter. In case of water at sea level and 392 F D 1 so L g x 1000.
Type in your own numbers in the form to convert the units. Successful scientists use the factor label method also called dimensional analysis. Type in your own numbers in the form to convert the units.
How many deci liter in 1 gram sugar. Gram g is a unit of Weight used in Metric system. Grams To Liters 1 liter l 1000 gram g.
GRAMS LITERS To convert between grams and liters you must always use 224 Litersmol and the molar mass. Liter l is a unit of Volume used in Metric system. Liter l is a unit of Volume used in the Metric system.
Im stuck on my hw which is due tomorow. Converting between Liters and Moles using the Factor Label Method This is the method of choice since you can use it to convert between any units mols to grams molecules to mols etc as long as you know the conversion factor. 1 cubic meter is equal to 1000000 grams or 1000 liter.
To convert liters to grams multiply the given number by 1000. Thank you for your help. I just need the formula to convert litres into grams.
1 liter l 1000 gram g. Liter l is a unit of Volume used in Metric system. A liter consists of 1000 milliliters and each milliliter is equal to 1 gram.
Note that rounding errors may occur so always check the results. Grams and moles are two units to express the amount of matter in a sample. This 224 Litersmol is only allowed at STP standard temperature pressure.
Use this page to learn how to convert between liters and grams. Shows how to convert grams of a substance to litres at STP. We assume you are converting between deciliter and gram sugar.
This calculator finishes the topic started in Convert moles to liters and liters to moles calculator. Whether youre converting from moles to grams moles to volume or moles to particles atoms or molecules use this quick guide to remind you of how to do each type of mole conversion. In a general chemistry class you usually end up having to perform a lot of conversions involving moles mol.
Solution for The average translational kinetic energy for a molecule Erans is given by the following equation. Interscale kinetic energy transfer in chemically reacting compressible isotropic turbulence - Volume 912.
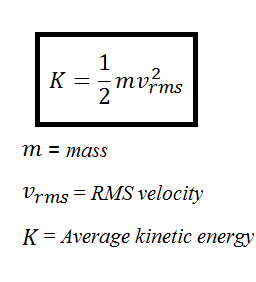
Average Kinetic Energy Temperature Of A System Video Lesson Transcript Study Com
It contains plenty of.
Formula for kinetic energy chemistry. Where the four-velocity of a particle is. I have read 2 formulas for kinetic energy of 1 mol of gas - 32 RT and 12fRT. Using our equation we can determine the kinetic energy of the sphere.
KE05times mtimes v2 where KE is kinetic energy in joules m is mass in kilograms and v is velocity in meters per second. So the sphere has a total kinetic energy of 2125 J. Your answer should always be stated in joules J which is the standard unit of measurement for kinetic energy.
K average kinetic energy per molecule of gas J. U α d x α d τ displaystyle u alpha frac dx alpha dtau and. The formula for the kinetic energy of a gas defines the average kinetic energy per molecule.
For extremely high-speed particles it yields values that are too small. Any help to gain insights into the derivation of this formula would be helpful. To figure the total kinetic energy you multiply the average kinetic energy by the number of molecules you have which is nNA where n is the number of moles.
Where m is the mass of the molecule. As an example to illustrate kinetic energy lets say that a 17 kg sphere is moving in a straight line with a velocity of 5 ms. I read from a book that average kinetic energy is equal to 3 k T 2 where k is Boltzmanns constant and T is the kelvin temperature.
Big things moving fast have the most energy the most ability to shove other things or knock them over etc. Kinetic energy is the energy that occurs in an object in motion. Kinetic energy is typically measured in units of Joules and 1 Joule is equal to 1 kilogram-meters squared per second squared.
Translational kinetic energy of a body is equal to one-half the product of its mass m and the square of its velocity v or 12mv2. 12 mv2 where m stands for mass and v stands for velocity. The formula for kinetic energy of an object is K 12mv2.
If the particle has momentum. The equation for kinetic energy is 1 K E 1 2 m v 2 where KE is kinetic energy m is mass and v is velocity. It is equivalent to 1 kg m 2 s 2.
KE ½ 17kg 5ms² 2125 J. This chemistry video tutorial explains how to calculate the average kinetic energy of a gas and the root mean square velocity as well. The Kinetic energy is articulated in Kgm 2 s 2.
I dont know how the formula was derived. For a simple monoatomic gas like helium or neon the only motion that the atoms can do is to move from one place to another in a straight line until they bump into something else such as another atom or molecule86 This kind of motion is called translational motion and is directly linked to the kinetic energy of the atom or molecule through the relationship KE 12 m vbar2 32 kT where vbar is the average velocity of all of the molecules in the population87 m is the mass k is a. The formula for the energy of motion is.
The formula for calculating kinetic energy KE is KE 05 x mv 2. τ displaystyle tau is the proper time of the particle there is also an expression for the kinetic energy of the particle in general relativity. Are we supposed to memorize and know how to solve for average kinetic energy.
Underneath are questions on Kinetic energy which aids one to understand where they can use these questions. NAk equals R the universal gas constant so this equation becomes the following. The kinetic energy is measured in Joules J and the temperature is measured in Kelvin K.
The equation is not on the equation sheet You do not have the required permissions to view the files attached to this post. This formula is valid only for low to relatively high speeds. The kinetic energy of the translational motion of an ideal gas depends on its temperature.
This definition should make sense. Kinetic Energy Solved Examples. Here m stands for mass the measure of how much matter is in an object and v stands for the velocity of the object or the rate at which the object changes its position.
By signing up youll get thousands of step-by-step. Potential energy is energy that comes from position and a force. Kinetic energy formula is used to compute the mass velocity or kinetic energy of the body if any of the two numerics are given.
The formula for kinetic energy is KE. But if I equate them then f comes out to be 3. But f is different of mono di polyatomic gases.
Energy is usually divided into two types by chemists.